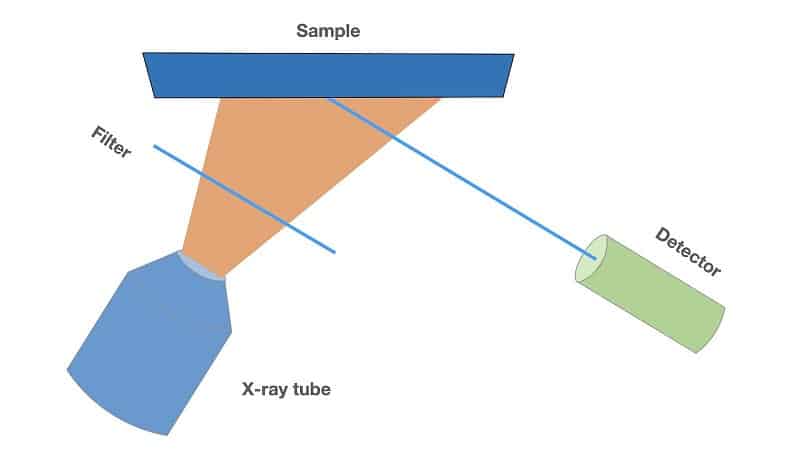
X-ray fluorescence analysis is based on the study of the characteristic fluorescence spectrum obtained by exposure the sample with X-rays. Schematic diagram of the XRF analyzer is shown in Fig.1.
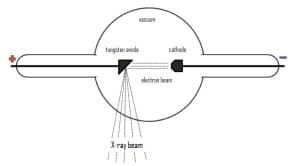
X-ray tubes are used as a radiation source in XRF, which are a vacuum chamber in which an anode and a cathode are located. The cathode, when heated, as a result of thermionic emission, emits electrons. Accelerated by the applied voltage, the electrons collide with the anode, and in the process of deceleration generate X-rays (Fig. 2).
The filter located between the sample and the tube is used to correct the spectrum of the initial X-ray beam in accordance with the analytical task. The radiation scattered from the sample enters the detector, which decomposes it into a spectrum. As detectors in energy dispersive XRF are used proportional chambers or solid-state detectors.
They work on the same principle: an incident quantum excites a sufficiently large number of atoms, generating a signal in the electrical circuit that is proportional to its energy.
Physical foundations of XRF
X-ray fluorescence occurs as a result of the absorption of an X-ray quantum by an atom, which knocks out an electron from the inner electron shells of the atom. The formed vacancy is quickly occupied by an electron from the outer shells, passing into a state with a lower energy (Fig. 3).
This, according to Bohr's postulates, is accompanied by the emission of a quantum with an energy equal to the difference between the energies of the initial and final states of the electron. Thus, the spectrum of radiation scattered from the sample will contain particularly intense lines at energies corresponding to the difference between the energies of the electronic states in the atom.
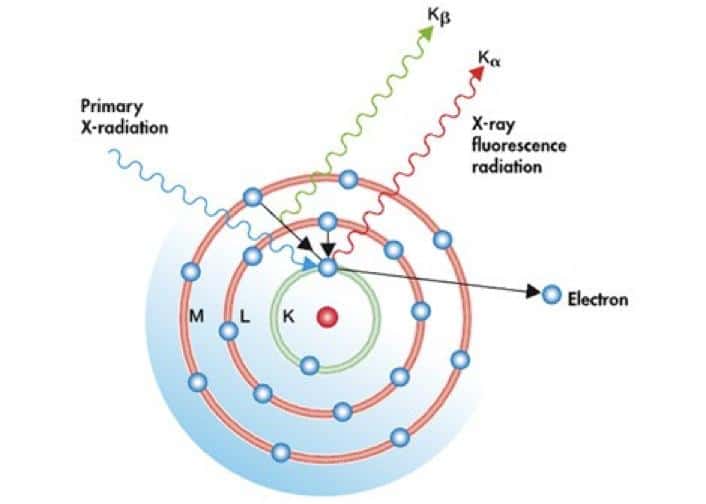
The energies of possible electronic transitions in an atom are unique for each element of the periodic table, since the energies of quantum states depend on the element's atomic number. This dependence is described by Moseley's law:
-300x86.png)
Z is the atomic number, A and b are constants depending on the series to which the line belongs.
Thus, simply from the presence of certain lines in the characteristic spectrum, one can judge the presence of a particular element in the sample under study.
Quantitative analysis
To carry out a quantitative analysis, it is necessary to establish the relationship between the intensities of the characteristic lines in the spectrum and the concentrations of elements in the sample.
To do this, one can use an empirical approach by comparing the line intensities in the obtained spectrum with the intensities of the same characteristic lines in the spectra of standards – samples with a known composition. Such an approach, however, requires the presence of similar composition standards for comparison.
A more modern approach is the method of fundamental parameters, based on the construction of a theoretical model of the interaction of X-rays with material to obtain equations relating the intensity of fluorescent lines to the elemental composition of the sample.
This approach allows, after a one-time calibration of the device, to calculate the concentrations of elements in the samples under study without the need for standards.
For more information about XRF technology, please visit the Elvatech company website.










